Microwave heating has significant advantages over conventional ohmic or ionic heating for subsurface removal of hydrocarbons. At frequencies below 1 to 2 GHz, referred to as low frequency or RF heating, the charge carrier for energy transfer in rocks is the interstitial water or groundwater that contains dissolved ions. This effect is shown in experiments commonly performed in grade school classes where electrodes placed in deionized water cannot transmit an electrical current until salt is added providing a charge carrier.
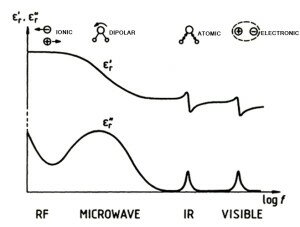
For subsurface heating by low frequency methods, once groundwater reaches the boiling point and evaporates, there is not a pathway to conduct electricity into the rock. The low frequency antenna becomes a heater and thermal conduction is relied upon to heat the rock. The only problem is that the rock is an insulator or poor conductor of thermal energy resulting in an inefficient process.
Microwave heating relies on dielectric heating where molecules rotate in an alternating electrical field causing friction and subsequently heating. At 2.45 GHz, the frequency of a microwave oven, a molecule will rotate 4.9 billion times a second. Microwave power directly couples into the water and hydrocarbons because of their dielectric properties resulting in efficient heating. Think of your microwave oven at home where the microwaves pass through the ceramic dish and couple into the water contained in the food. Once the food heats up, the ceramic dish becomes hot due to conduction. The ceramic dish is composed of material common with many types of rocks. The efficiency of heating food in a microwave oven versus a conventional conduction oven is well know. Uneven heating, a problem with microwave cooking, is a big advantage in heating hydrocarbons in the subsurface.